Health News Roundup: Uganda begins Ebola vaccinations; FDA approves Pfizer's treatment
Following is a summary of current health news briefs.
Tall people may be more prone to varicose veins
Standing above the crowd puts people at greater risk of developing varicose veins, a large genetic study suggests. Varicose veins are swollen, twisted veins that can be seen just under the surface of the skin, usually in the legs. More than 30 million people in the U.S. have varicose veins. Although the condition is often dismissed as nothing more than a cosmetic nuisance, it can cause moderate pain and has been linked to the more serious side effect of deep vein thrombosis, or blood clots in the deep veins in the body.
Pharmacies still blocking U.S. teens looking for emergency contraception
Teens seeking to buy emergency contraception at pharmacies continue to face significant roadblocks, a new U.S. study suggests. Researchers checking on the accessibility of the "morning after pill" at pharmacies in four Southwestern states found that just 28 per cent made it simple and straightforward for teens to purchase the emergency contraception, according to the results published in the Journal of Adolescent Health.
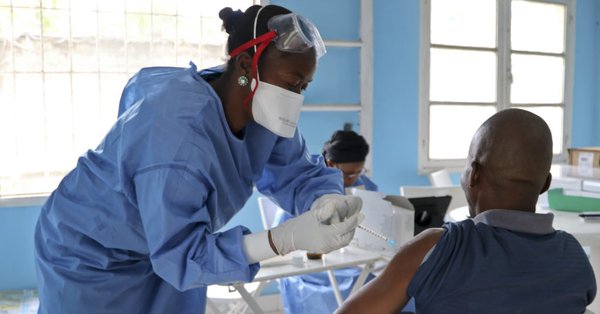
Uganda begins Ebola vaccinations amid Congo transmission fears
Uganda says it will start to vaccinate some of its health workers against Ebola on Monday, amid fears that the viral haemorrhagic fever could spread from the Democratic Republic of Congo which is battling an outbreak. The East African country has suffered regular outbreaks of Ebola and Marburg over the years, both high-fatality viral haemorrhagic fevers.
FDA approves Pfizer's treatment for certain lung cancer patients
Pfizer Inc said on Friday the U.S. Food and Drug Administration has approved its lung-cancer treatment for patients with a specific gene mutation who had been previously treated for an aggressive form of the disease. The treatment, Lorbrena, is designed to treat advanced non-small cell lung cancer in patients with a mutation of the ALK gene, who have relapsed after being treated with ALK inhibitors, such as Pfizer's Xalkori.
Novartis abandons effort for U.S. approval of biosimilar rituximab
Novartis International AG said on Friday that its Sandoz division is abandoning an effort to gain U.S. regulatory approval for a biosimilar of rituximab, a medication used to treat certain cancer and rheumatoid arthritis. The decision comes after the U.S. Food and Drug Administration (FDA) sought additional information to support the company's application for the drug, which is approved already in the EU, Switzerland, Japan and Australia, the company said in a statement.
FDA approves AcelRx Pharma's opioid pain drug
The U.S. Food and Drug Administration on Friday approved AcelRx Pharmaceuticals Inc's opioid-based treatment for pain to be used under strict medical supervision, with the agency's chief highlighting reasons for the approval in a rare move. The regulator's decision comes as the U.S. opioid crisis reaches epidemic levels, claiming lives of an estimated 130 Americans a day on average, according to the U.S. Centers for Disease Control and Prevention.
Trevena opioid painkiller fails to win FDA approval, shares plunge
Trevena Inc shares plunged 45 per cent on Friday after the U.S. Food and Drug Administration declined to approve its opioid injection for managing acute pain, citing inadequate safety data. The widely expected rejection comes after a panel of experts to the FDA voted against the approval of the drug due to lack of safety data and potential for abuse, at a time when opioid addiction in the country has reached epidemic proportions.
FDA panel recommends Sage's postpartum depression treatment
An advisory panel to the U.S. Food and Drug Administration on Friday recommended Sage Therapeutics Inc's experimental treatment for postpartum depression, saying the benefits of the drug outweighed risks. The panel voted 17-1 in favour of the injectable treatment, Zulresso, which aims to treat major episodes of depression during pregnancy or within four weeks of delivery.
E-therapy may help ease insomnia
People with insomnia who receive a digitized version of cognitive behavioural therapy (CBT) as part of their treatment may find more symptom relief than those who only receive tips to improve their sleep routines, a recent experiment suggests. Researchers randomly assigned 1,700 insomnia patients to receive either digital CBT or so-called sleep hygiene education designed to improve bedtime routines and encourage avoidance of substances like caffeine and alcohol that can interfere with sleep.
(With inputs from agencies.)
- READ MORE ON:
- Food and Drug Administration
- Patient
- Novartis
- Novartis India Ltd.
- Sanofi
- Clinical trial
- Company
- Pembrolizumab
- Route of administration
- Drug Enforcement Administration
- Behavior
- Dialectical behavior therapy
- Cognitive behavioral therapy
- Medication
- lung-cancer treatment
- FDA approval
- Southwestern United States
- FDA panel
- Pfizer's treatment
- Drug Administration










